Why Is Water Less Dense as a Solid
Solid water or ice is less dense than liquid water. When water freezes water molecules form a crystalline structure maintained by hydrogen bonding.

Matter Notes Pre Ap Biology Science Notes Nursing School Notes School Organization Notes
The density of water is roughly 1 gram per milliliter but this changes with temperature or if there are substances dissolved in it.

. Why is it so important that solid water is less dense than liquid water. Also as the temperature increases the density of the water starts decreasing. This is why ice floats.
Solid water or ice is less dense than liquid water. The a lattice structure of ice makes it less dense than the freely flowing molecules of liquid water enabling it to b float on water. It has less molecules than an equal volume of liquid water.
The density of water is quite unique as compared to other liquid substances. What happens as water freezes. When it melts framework collapses and the water molecules pack closer together making liquid water more dense.
And this is less dense. The liquid phase is less dense than the solid phase but more dense than the gas phase and the gas phase is less dense than either the solid or. For all substances density changes with temperature.
The reason is hydrogen bonds. This is more dense more dense. The mass of material does not change but the volume or space that it occupies either increases or decreases with temperature.
The water molecules are pushed farther apart compared to liquid water. When water freezes water molecules form a crystalline structure maintained by hydrogen bonding. Rather than the molecules packing more tightly together and creating a heavier.
Solid water or ice is less dense than liquid waterIce is less dense than water because the orientation of hydrogen bonds causes molecules to. Solid water or ice has less density than liquid water because of waters unique crystalline formation when frozen. W aters lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes.
Each molecule forms stable hydrogen bonds and creates a 3-D crystal. With most other liquids solidification when the temperature drops includes the lowering of kinetic energy between molecules allowing them to pack even more tightly than in liquid form and giving the solid a. Apr 21 2017 at 1633.
As you might expect water density is an important water measurement. Why is solid water less dense than liquid water. Solid water or ice is less dense than liquid water.
Water is made up of two hydrogen atoms and one oxygen atom. Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart which lowers the density. The ice crystals that form upon freezing rupture the delicate membranes essential for the function of living cells irreversibly damaging them.
This means ice floats on water. It gets less dense when it freezes because everything lines up and forms orderly crystals it has a high specific heat and is about the. Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart which lowers the density.
Why is ice less dense than water. In practical terms density is the weight of a substance for a specific volume. Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart which lowers the density.
When were talking about water when were talking about water when we go to the liquid state when we go from liquid water to solid ice to solid ice we actually get we actually get less dense. Hydrogen bonds hold water molecules in place in solid phase. Water is fun stuff.
Hydrogen bonding makes ice less dense than liquid water. When water is frozen into ice the change in temperature creates excess hydrogen bonds between water molecules that increase the space between the molecules. As water cools so the hydrogen bonds align to cause the water molecules to become aligned and each molecule takes up more space so the solid is less dense.
The water molecules are pushed farther apart compared to liquid water. As water cools so the hydrogen bonds align to cause the water molecules to become aligned and each molecule takes up more space so the solid is less dense. Why is water less dense as a solid than a liquid.
Density is the mass per unit volume of a material. Structure of ice is a regular open framework of water molecules arranged like honeycomb. Ice is less dense as a solid than as a liquid ice floats Liquid water has hydrogen bonds that are constantly being broken and reformed.
Objects like apples wood and sponges are less dense than water. The detrimental effect of freezing on living organisms is caused by the expansion of ice relative to liquid water. Given that ice is less dense than water why doesnt it sit completely atop water rather than slightly submerged.
So this right over here is less dense. Waters lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes. The water molecules are pushed farther apart compared to liquid water.
Waters lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes. The additional space created reduces the density of the water as it freezes making ice less dense than water. For other substances as the liquid cools so the molecules jostle around and take up less space so the solid is more dense.
The water molecules are pushed farther apart compared to liquid water. For other substances as the liquid cools so the molecules jostle around and take up less space so the solid is more dense. The water molecules are pushed farther apart compared to liquid water.
Begingroup Possible duplicate of Density of Solid States of Compounds endgroup Floris. Waters lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes. Why is Water Less Dense as a Solid.
Frozen water forms a crystal-like lattice whereby molecules are set at. Water is unusual in that its maximum density occurs as a liquid rather than as a solid. Waters lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes.
Ice is less dense than liquid water which is why your ice cubes float in your glass. What objects are less dense than water. The density of water is maximum at 4 as the temperature decreases the density also decreases.

Interesting Facts About Water Water Facts Facts Water Molecule

Pin On The Pensive Sloth S Pins

What Is Decantation Definition And Examples Chemistry Teaching Chemistry Chemistry Science Diagrams

Because Of Hydrogen Bonding Water Is Less Dense As A Solid Than It Is As A Liquid Consequently Ice Floats Chemistry Education Faculties

Concept Cartoon This Concept Cartoon Titled Added Energy Could Be Used To Assess Students On Their Understanding Of Particle Understanding Particles Energy

Phases Of Matter Gas Liquids Solids Solid Liquid Gas Liquid Gas

Pin By Eli Solomon On Properties Of Water Surface Tension Molecules Surface

Physical Properties Of Matter Discussion Questions Task Cards Digital Slides Physical Properties Of Matter Properties Of Matter Physical Properties

Pin On Atoms Elements And The Periodic Table

Starting The Year Off Right Science Ideas For The First Week Science Elementary Science Teaching Science

Properties Of Water Science Project Science Projects School Projects Projects

Surfer S Tension By 4mule8 Best Puns Surfer 3d Printing

Properties Of Water Lesson Plan A Complete Science Lesson Using The 5e Method Of Instruction Water Lessons Science Lessons Chemistry Lesson Plans

Ice Floats Because Solid Water Is Less Dense Than Liquid Water
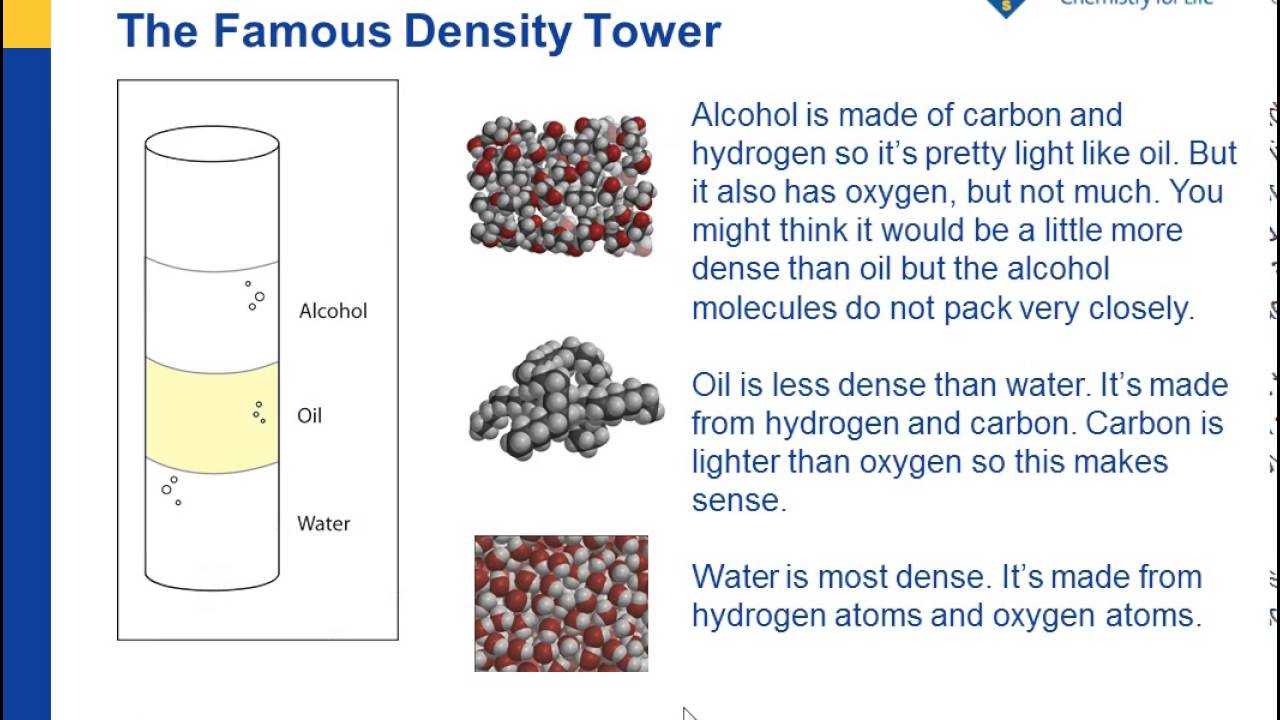
Density Sink And Float For Liquids Chapter 3 Density Middle School Chemistry Middle School Chemistry Teaching Chemistry Chemistry

Pin By Merium Alvi On Polymath Renaissance Person Science Experiments Kids Elementary Science Method Teaching Science



Comments
Post a Comment